How do I convert wavelength to frequency?
How do I convert wavelength to frequency?
What is the formula to find the wavelength of a frequency?
λ = v/f. The Wavelength is expressed in m, velocity is expressed in m/s, frequency is expressed in Hz.
How is wavelength λ related to frequency ν )?
Wavelength is related to energy and frequency by E = hν = hc/λ, where E = energy, h = Planck’s constant, ν = frequency, c = the speed of light, and λ = wavelength. Wavelength the distance between any given point and the same point in the next wave cycle.
What is the wavelength at frequency of 900 Mhz?
0.33 m. Download the app to view unlimited solutions on app.
What is frequency * wavelength?
Waves have a variety of features that can be used to define them. Two such properties are wavelength and frequency. As we’ll see below, the link between wavelength and frequency is that the frequency of a wave multiplied by its wavelength yields the wave’s speed.
What is frequency equal to wavelength?
They are all related by one important equation: Any electromagnetic wave’s frequency multiplied by its wavelength equals the speed of light. FREQUENCY OF OSCILLATION x WAVELENGTH = SPEED OF LIGHT.
How do I calculate frequency?
To calculate frequency, divide the number of times the event occurs by the length of time. Example: Anna divides the number of website clicks (236) by the length of time (one hour, or 60 minutes). She finds that she receives 3.9 clicks per minute.
What is the wavelength of light waves with frequency is 5.0 x10 * 14 Hz?
Hence, the wavelength will become 4000A˚.
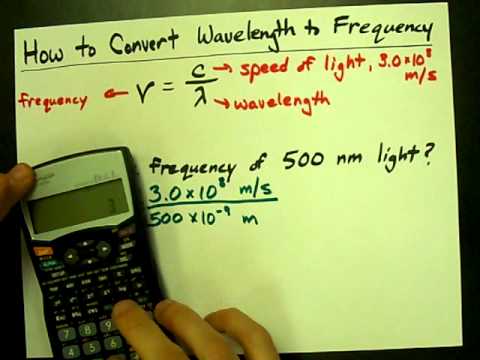
What is the frequency of green light that has a wavelength of 500 nm?
Solution: Given, the wavelength of the photon particle = 500 nm. In order to calculate the frequency of the photon particle, we use the formula given above. The frequency of the wave is equal to 6×10-4 Hz.
What is 1 lambda?
Lambda (written λ, in lowercase) is a non-SI unit of volume equal to 10−9 m3, 1 cubic millimetre (mm3) or 1 microlitre (μL). Introduced by the BIPM in 1880, the lambda has been used in chemistry and in law for measuring volume, but its use is not recommended.
What is ν in our wave equations?
The frequency, represented by the Greek letter nu (ν), is the number of waves that pass a certain point in a specified amount of time. Typically, frequency is measured in units of cycles per second or waves per second.
How is wavenumber n related to frequency ν )?
The wavenumber is just another measure of the frequency ν. It is just the frequency times a constant (the speed of light). Since frequency is a measure of the energy of the absorption, the wavenumber is also a measure of the energy.
What is the wavelength of 500 MHz?
An electromagnetic wave has a frequency of 500 MHz and a wavelength of 60 cm.
What is the wavelength of 300 MHz?
The standard divisions are: Ultra high frequency (UHF): Frequencies of 300 MHz to 3 GHz, hence wavelengths of 10 cm to 1m. This overlaps with the microwave region.
What is the frequency of a 700 nm wave?
In the same way, we calculate that light with a wavelength of 700 nm has a frequency of 4.29 × 1014 Hz.
Is frequency and Lambda same?
f is the wave frequency, λ is the wavelength. The relation between the frequency and wavelength can be derives using the formulas for these two quantities.
How many Hz is a frequency?
Frequency is the rate at which current changes direction per second. It is measured in hertz (Hz), an international unit of measure where 1 hertz is equal to 1 cycle per second. Hertz (Hz) = One hertz is equal to one cycle per second. Cycle = One complete wave of alternating current or voltage.
What is frequency and wavelength 9?
1. Wavelength : The distance travelled by the wave during one complete oscillation is called wavelength of the wave. 2. Frequency : The number of oscillations made by the wave in one second is called the frequency of the wave.
What is the wavelength of light waves with frequency is 5.0 x10 * 14 Hz?
Hence, the wavelength will become 4000A˚.
How to convert Ångström to hertz?
Answer and Explanation: Given- The wavelength is λ=4000 Angstrom=4000×10−10 m=4000×10−8 cm λ = 4000 Angstrom = 4000 × 10 − 10 m = 4000 × 10 − 8 cm . Thus, the frequency is 7.5×1014 Hz 7.5 × 10 14 Hz .
What is the frequency of a light wave with a wavelength of 410 nm wave?
Violet light has a wavelength of 410 nm. To calculate the energy of one photon of violet light, we need to know its frequency (in hertz) and its energy (in joules). Violet light has a frequency of 410,000 hertz and an energy of 1.0 joules.
How do you calculate frequency?
To calculate frequency, divide the number of times the event occurs by the length of time. Example: Anna divides the number of website clicks (236) by the length of time (one hour, or 60 minutes).
