How do you find number of neutrons?
How do you find number of neutrons?
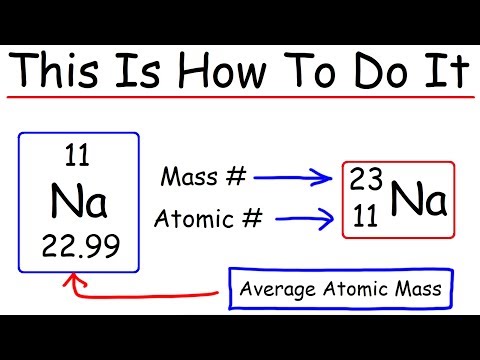
You can simply subtract the atomic number from the mass number in order to find the number of neutrons.
How do I find the number of protons?
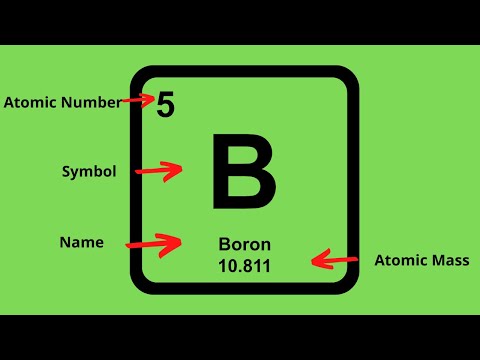
Finding the Number of Protons The number of protons in an atom is equal to the atomic number of the element. For example, let’s use oxygen. According to the periodic table, oxygen has the atomic number eight. The atomic number is located above the element’s symbol.
How do you find the electrons?
How do you find neutrons in electrons?
The easiest way to find the number of protons, neutrons, and electrons for an element is to look at the element’s atomic number on the periodic table.
Where do you find neutrons and protons?
Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Protons and neutrons are in the center of the atom, making up the nucleus. Electrons surround the nucleus. Protons have a positive charge.
What is the easiest way to find protons?
How many electrons are in protons?
In other words, a neutral atom must have exactly one electron for every proton. If a neutral atom has 1 proton, it must have 1 electron.
Are protons and neutrons equal?
Statement 1: Number of neutrons is always greater than or equal to the number of protons in any given atom except hydrogen.
How do you find the number of electrons in a charge?
The charge on the ion tells you the number of electrons. If the charge is positive, subtract that number from the atomic number to get the number of electrons. You have more protons. If the charge is negative, add the amount of charge to the atomic number to get the number of electrons.
Where can we find neutrons in an atom?
A neutron is a subatomic particle found in the nucleus of every atom except that of simple hydrogen. The particle derives its name from the fact that it has no electrical charge; it is neutral. Neutrons are extremely dense.
How do you find the number of protons in an atom or ion?
The number of protons is equal to the atomic number of the element. To find the number of protons, find the atomic number of the element in the periodic table. Forming an ion does not change the number of protons in an atom.
What is the number of a protons?
The number of protons in an atom is called its atomic number (Z). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms.
