How do you write protons?
How do you write protons?
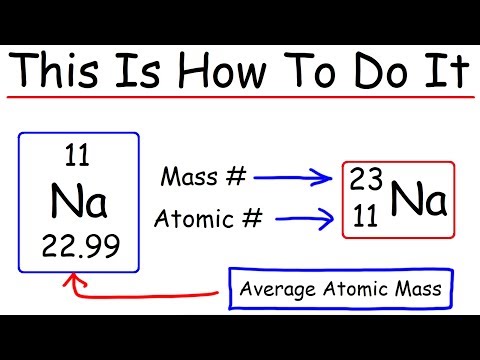
Are protons A or Z?
The term atomic number, conventionally denoted by the symbol Z, indicates number of protons present in the nucleus of an atom, which is also equal to the number of electrons in an uncharged atom. The number of neutrons is represented by the neutron number (N).
What element is a proton?
Atomic Number
| Name | Protons | |
|---|---|---|
| Hydrogen | 1 | 1 |
| Helium | 2 | 4 |
| Lithium | 3 | 7 |
| Beryllium | 4 | 9 |
What is the symbol of electron and proton?
| Subatomic particle | mass | symbol |
|---|---|---|
| Electron | 1/1840 of the mass of Hydrogen(H) atom | e– |
| Proton | 1.6 x 10–27 g | p |
| Neutron | 1.6 x 10–27 g | n |
What is a proton in short?
A proton is a subatomic particle found in the nucleus of every atom. The particle has a positive electrical charge, equal and opposite to that of the electron.
Where is proton written?
One or more protons are present in the nucleus of every atom.
Why is proton Z?
The discovery of the neutron makes Z the proton number Since Moseley had previously shown that the atomic number Z of an element equals this positive charge, it was now clear that Z is identical to the number of protons of its nuclei.
Does Z mean electrons?
The atomic number (Z) of an element is the number of protons in the nucleus of each atom of that element. This means that the number of protons is the characteristic which makes each element unique compared to all other elements.
What is A and Z in atom?
Z = atomic number = number of protons in the nucleus = number of electrons orbiting the nucleus; A = mass number = number of protons and neutrons in the most common (or most stable) nucleus.
What is one proton called?
Protons are made up of fundamental particles called quarks (two up quarks and a down quark) held together by gluons. The nucleus of the simplest atom, Hydrogen (H), is a single proton.
Why is it called proton?
The proton was discovered by Ernest Rutherford in the early 1900’s. During this period, his research resulted in a nuclear reaction which led to the first ‘splitting’ of the atom, where he discovered protons. He named his discovery “protons” based on the Greek word “protos” which means first.
What is proton name?
Based on Wilhelm Wien’s theory, who in 1898 discovered the proton in streams of ionized gas, Rutherford postulated the hydrogen nucleus to be a new particle in 1920, which he called proton. Rutherford named it the proton, from the Greek word “protos,” meaning “first.”
Is p the symbol of proton?
A proton is a subatomic particle, symbol p or p+, with a positive electric charge of +1e elementary charge and a mass slightly less than that of a neutron.
What is the symbol of proton and neutron?
Neutrons
| Particle | Symbol | Location |
|---|---|---|
| proton | p+ | inside the nucleus |
| electron | e− | outside the nucleus |
| neutron | n0 | inside the nucleus |
What symbol is used for electrons?
Standard Model of elementary particles. The electron (symbol e) is on the left.
Is a proton an atom?
A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) and a mass of 1 atomic mass unit (amu), which is about 1.67×10−27 kilograms.
What is a proton example?
Examples of Protons The nucleus of a hydrogen atom or the H+ ion is an example of a proton. Regardless of the isotope, each atom of hydrogen has 1 proton; each helium atom contains 2 protons; each lithium atom contains 3 protons and so on.
Why is a proton positive?
A proton has positive charge of 1, that is, equal but opposite to the charge of an electron. A neutron, like the name implies, is neutral with no net charge. The charge is believed to be from the charge of the quarks that make up the nucleons (protons and neutrons).
How do you write protons and neutrons?
To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a superscript to the left of the atomic symbol.
How do you write protons neutrons and electrons?
How do you write neutrons?
The difference between the neutron number and the atomic number is known as the neutron excess: D = N − Z = A − 2Z. Neutron number is not written explicitly in nuclide symbol notation, but can be inferred as it is the difference between the two left-hand numbers (atomic number and mass).
What is a proton example?
Examples of Protons The nucleus of a hydrogen atom or the H+ ion is an example of a proton. Regardless of the isotope, each atom of hydrogen has 1 proton; each helium atom contains 2 protons; each lithium atom contains 3 protons and so on.
