How many valence electrons are in the atoms?
How many valence electrons are in the atoms?
1 Answer. The number of valence electrons that an atom has is equal to the group number of that atom.
What diagram shows the valence electrons of an atom?
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
What is the valence of an atom?
valence, also spelled valency, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Introduced in 1868, the term is used to express both the power of combination of an element in general and the numerical value of the power of combination.
What are valence electrons and an atoms valence?
A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair.
Where do you find valence electrons?
Valence electrons can be found by determining the electronic configurations of elements. Thereafter the number of electrons in the outermost shell gives the total number of valence electrons in that element.
How do you find the total number of valence electrons?
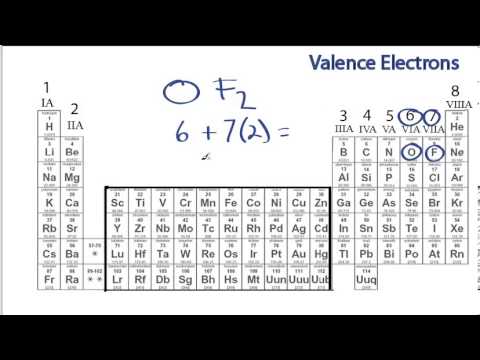
How do you draw valence electrons diagram?
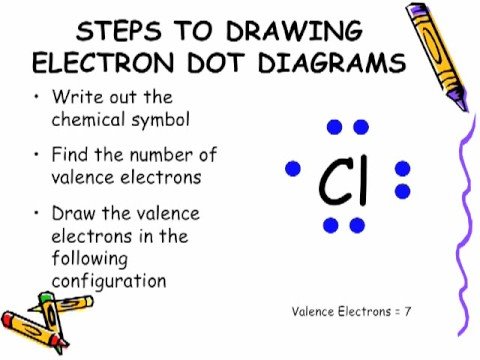
How do you draw a valence structure?
How to Draw a Lewis Structure
- Step 1: Find the Total Number of Valence Electrons. …
- Step 2: Find the Number of Electrons Needed to Make the Atoms “Happy” …
- Step 3: Determine the Number of Bonds in the Molecule. …
- Step 4: Choose a Central Atom. …
- Step 5: Draw a Skeletal Structure. …
- Step 6: Place Electrons Around Outside Atoms.
Where are the valence electrons in an atom quizlet?
Valence electrons are located in the outermost occupied shell of an atom. They are important because they play a leading role in determining the chemical properties of the atom.
What is valency example?
Valency is simply equal to the number of electrons gained, lost or shared by an atom of an element to achieve the nearest noble gas configuration. For example, the valency of sodium (Na) is 1, magnesium (Mg) is 2, Chlorine (Cl) is 1 etc.
What are examples of valence?
In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen. Phosphorus has a valence of 5 in phosphorus pentachloride, PCl 5.
What element has a valence electron of 4?
Carbon has 4 valence electrons.
What is an element with 3 valence electrons?
Gallium therefore has three valence electrons.
What is true valence electrons?
What is true of valence electrons? → They are always the lowest energy electrons of the atom.
Does the Bohr model show valence electrons?
Bohr models are used to predict reactivity in elements. Reactivity refers to how likely an element is to form a compound with another element. When looking at Bohr models, we look at its valence electrons (the electrons on the last energy level) to determine reactivity.
How do you find the valence electrons in a Bohr diagram?
What is an electron shell diagram?
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. This is sometimes called the Bohr, or the ‘solar system’, model.
